Fight for Copaxone -Teva pharmaceuticals-vs-generics

“If health is lost something is lost”. However, when a person suffers from a debilitating disorder, which damages wide range of abilities of a person, from physical to cognitive, this statement has to be restated as ‘if health is lost almost everything is lost’.
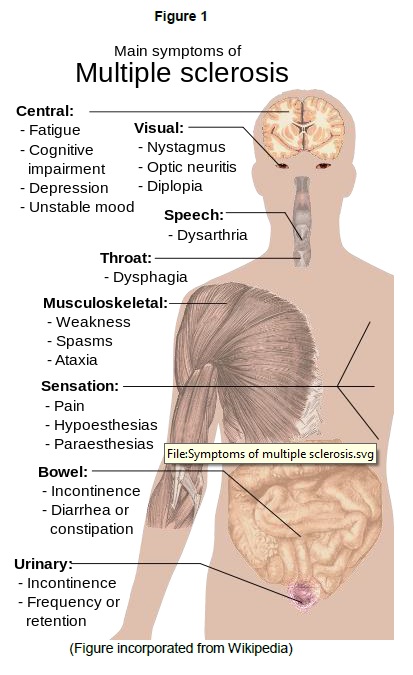
Multiple sclerosis (MS) is such a disabling disorder wherein the immune system attacks the protective myelin sheath that covers the nerves. This eventually disrupts the flow of information within the brain and between brain and the body. Figure 1 can explain the extent of damage MS can do to patients [1].
When a drug becomes available for such a malady, promising to reduce the suffering of patients, how hopeful the patients would be? And, when the cost of the drug is unaffordable, imagine the predicament of patients and their families. While some attempts ensue to bring it down to affordable cost, assume the eagerness of patients, and when it does not happen, think of their disappointment!
When a patient is going through such distress and pain, on the flip side, a perpetual, acquisitive war continues between the innovation companies and their generic competitors.
Has the whole purpose of innovation not been overlooked??!!
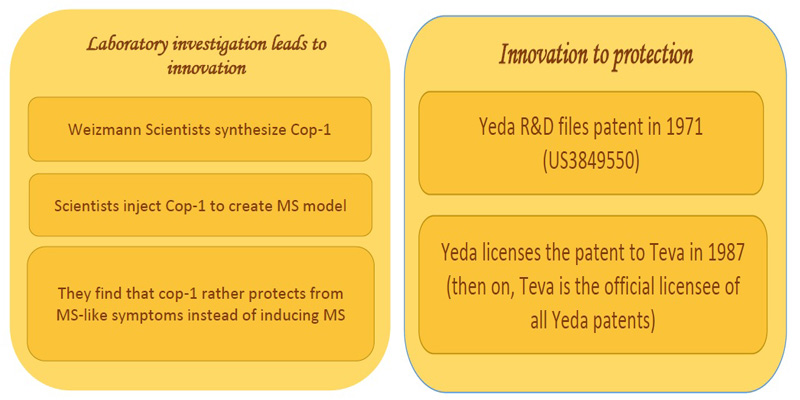
Investigation to innovation:
Scientists from the Weizmann institute of science synthesized several molecules known as copolymers. Copolymer-1 also commonly called GLATiramer is a random polymer of four amino acids found in myelin basic protein, namely Glutamic acid, Lysine, Alanine and Tyrosine. Myelin basic protein is important for myelination of nerves, which in turn is required for proper axonal conduction of electrical impulses. In an attempt towards creating animal models for studying multiple sclerosis, the scientists found that copolymer rather blocked the MS- like symptoms in mice [2].
Innovation to IP protection:
Yeda Research and Development, the technology transfer arm of Weizmann institute of sciences, filed a patent first in 1971 (US3849550). This was licensed to Teva pharmaceuticals Ltd, Israel, in the year, 1987. Then on, all the copolymer-1 related patents of Yeda were licensed to Teva [3].
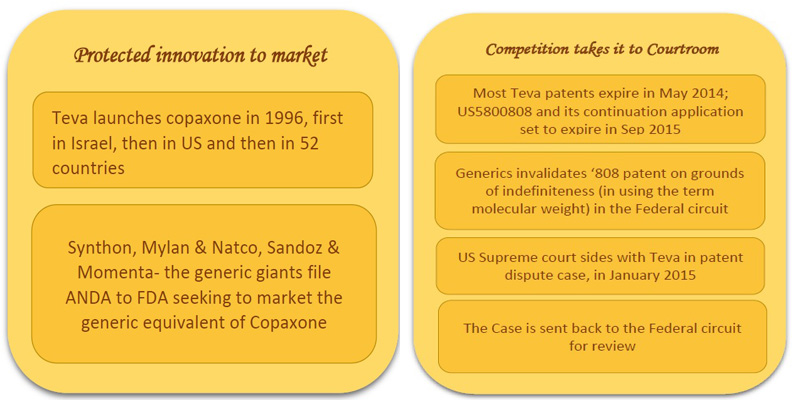
Protected Innovation to Market:
Teva launched Copaxone, a drug promising to reduce the frequency of relapses in MS patients, first in 1996, in Israel, then in 1997 in US and then widely in 52 countries [4]. The active ingredient is copolymer-1, or glatiramer acetate, which is a mixture of individual polymer molecules with different constituent ratios and thus different molecular weights.
Teva has been enjoying exclusive rights for marketing the drug till today. However, efforts by generic competitors such as Synthon, Mylan, Natco and Sandoz to bring in the cheaper generic versions into the market has encroached the isolated space Teva has been enjoying all the while. Synthon, Natco and Mylan, Sandoz and Momenta had already filed an Abbreviated New Drug Application (ANDA) to FDA for getting approval of the generic version of daily injecting 20mg/mL drug glatiramer [5]. Further, recently FDA has nodded to ‘Glatopa’, the generic version of Sandoz & Momenta, in April 2015. [6]
Several Teva’s patents expired by last May 2014. The generic competitors could have barged into the market but for the patent US5800808 which is due to expire in September 2015.
The generic giants had invalidated the ‘808 patent for its indefiniteness in using the term molecular weight in the federal circuit in the US. Indefiniteness refers to a patent claim that can have different meanings depending on the method used to measure it. The grounds for invalidation was that molecular weight could be calculated by at least three different ways – peak average molecular weight, number average molecular weight and weight average molecular weight [7] and the ‘808 patent claimed that ‘the process’ results in copolymer-1 having molecular weight from 5 to 9 KDa, thus resulting in ambiguity. However, in January 2015 the US Supreme Court favored Teva and reversed the lower court’s ruling. The case was sent back to federal circuit for further review [8,9]. In June 2015, a further blow on Teva’s spirits was due to the US courts rescindment of Teva’s patent [10].
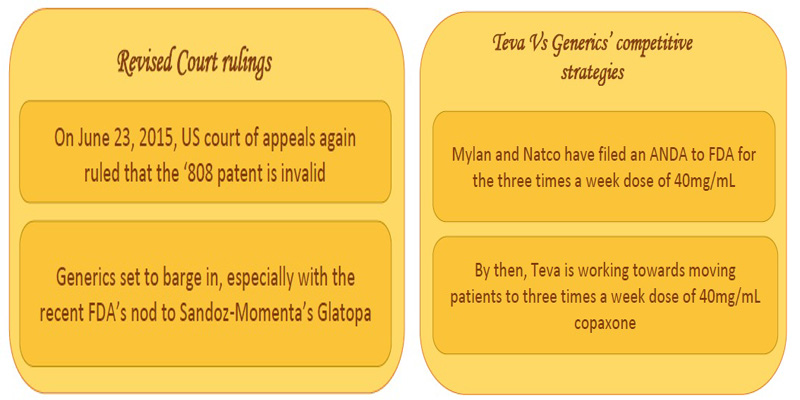
The Tussle continues:
In the meantime, Teva had been working strategically towards moving the patients from the current 20mg/mL daily subcutaneous dose of Copaxone to three times a week 40mg/mL dose of copaxone (US8232250, US8399413) [11,12].
However, the generic opponents had filed an ANDA for the three times a week 40mg/mL dose of Copaxone, as well [13, 14, 15]. Adding further to the competition is MAPI pharma’s patent (US8377885) on once a month dose of glatiramer acetate in its slow release form.
The story of the ‘product patent’ and ‘process patent’ in India:
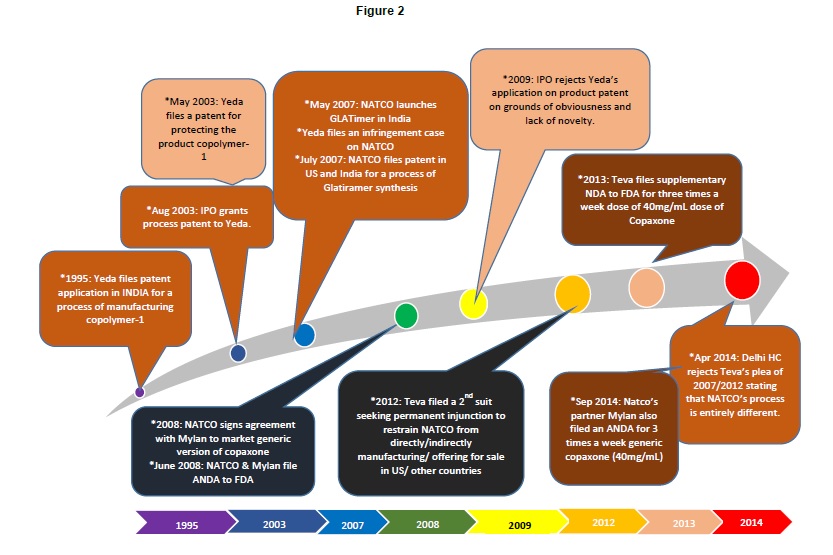
In India, it’s a whole new ball game. In 2003, Yeda filed an application no. 93/DEL/2003 in the Indian patent office towards a product patent for glatiramer acetate to extend their exclusive rights over Copaxone in India. Subsequently, Natco filed a pre grant opposition that led to rejection of the patent application in India on grounds of lack of inventiveness and non-obviousness [16] in 2009. Natco Pharmaceuticals is already selling GLATIMER, a generic version of copaxone at an affordable rate, in India. Yeda and Teva had filed a suit against Natco on grounds of infringement into their Indian Patent IN190759, a process patent claiming a process of manufacture of copolymer-1 [17]. However, the Delhi high court turned down Teva in April 2014, since the manufacturing process followed by NATCO was different from that of Teva’s process to manufacture Copaxone [18]. Further, Natco has an US Patent 8993722 and an Indian patent 264362 in which the procedure of glatiramer acetate preparation involves different reaction temperature and time and an additional step as compared to Teva’s patent.
DexPatent has illustrated these events relating to India in Figure 2.
Further, NATCO’s effort to export prefilled syringes with copolymer-1 to Mylan Inc. for distribution in USA [19] could also be a smooth sail with the invalidation of Teva’s ‘808 in June 2015.
Are innovations too-pricy?
An analysis from the Bloomberg says [20], the prices of old MS drugs are equivalent to that of the new ones. For e.g., MS drug copaxone costed less than $10,000 per year when it was launched in 1996, but in 2013 their prices hiked up to $50000-60000, equivalent to the newly launched drugs.
Patents besides promoting innovation and giving fair benefit to the innovators, should also benefit all the community. Can that be achieved through better effective IP policies? Do we also need intervention of the authorities in fixing affordable prices for at least life-changing drugs? In this context, is India’s IP policy for drugs that is generally in the interest of patients [21, 22] congenial to the patent community, as well?
Need to ponder upon this awhile!
The authors, Dr. Lalitha & Sivaranjani are Patent Scientists at DexPatent.
For any comments/ feedbacks please write to lalitha@dexmail.in or info@dexpatent.com











0 Comments