Significance of Section 3(d) in filing pharmaceutical patents in India.

DexPatent summary of the case:
1. Pfizer has a patent in-force in India for Tofecitinib, an arthritis drug, already.
2. Pfizer filed another patent in India in 2003, for an enantiomer of Tofecitinib.
3. The first application of this was filed with a priority date of May 31st 2001.
4. IPO rejected the application based on the fact that it’s not novel under section 3d citing Pfizer’s own prior art that was published in November 22, 2001.
5. Pfizer argued that the ‘cited reference’ is not a prior art as it is published after May 31st (i.e. on November 22nd, 2001)
6. But IPO defended based on section 13(1) b, i.e., although the publication date for the Pfizer’s ‘prior art’ is November 22 2001, the priority date is Dec 10, 1999 and hence is indeed a prior art.
Read the full story compiled below………
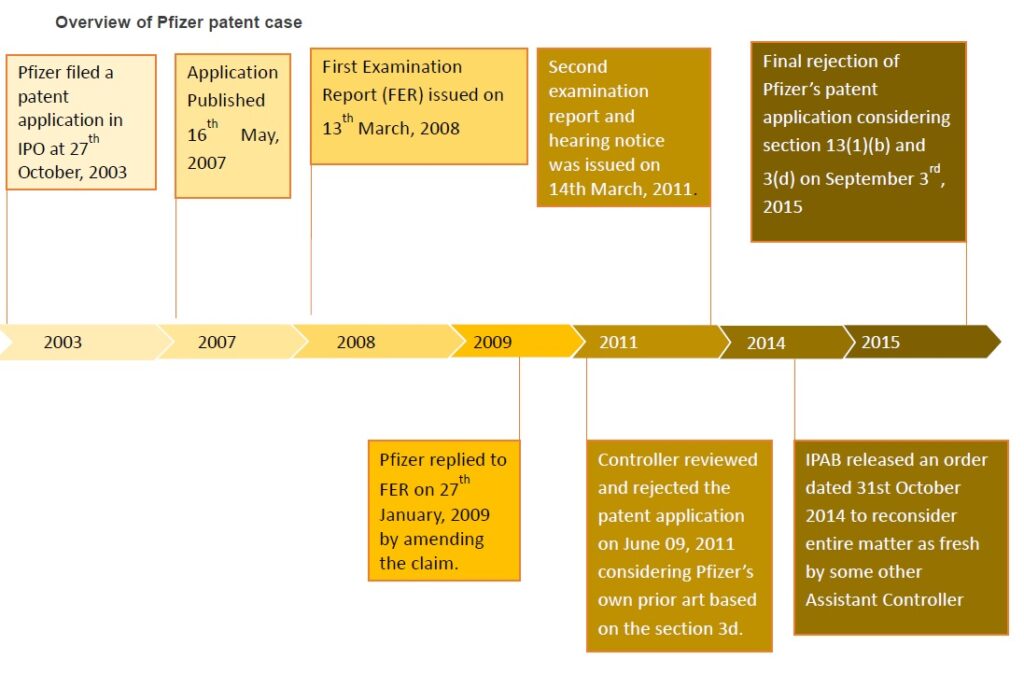
On September 03, 2015, Indian patent office rejected Pfizer’s patent application for the arthritis drug, Tofacitinib (also known as Xeljanz and Jakvinus) is an approved drug for the Pfizer treatment of rheumatoid arthritis (RA) in the USA and some other countries. Pfizer filed a patent application entitled “CHIRAL SALT RESOLUTION” (application number: 991/MUMNP/2003) in Indian Patent office (IPO) on 27th October, 2003 which is the national phase application of WO2002096909 filed on 29th May, 2002. The application was published on 16th May, 2007. The present application is an improved chemical formulation of Tofacitinib. The base form of Tofacitinib had already been patented in India.
Initially, the application was examined under Section 12 and 13 of Patents Act and first examination report was issued on 13th March, 2008 by Dr. Amarendra Samal, Asst. Controller of Patents & Designs. The applicant replied to the first examination report on 27th January, 2009. Then second examination report and hearing notice was issued on 14th March, 2011. In the hearing, amended patent application was reviewed and rejected on June 09, 2011. The controller stated in the order that claims 1 & 2 were not novel considering Pfizer’s own application WO0142246, as closest prior art based on the section 3d of THE INDIAN PATENTS ACT, 1970.
Then Pfizer challenged the order of IPO at Intellectual Property Appellate Board (IPAB). During the challenge, contention put forward by Pfizer was that the controller didn’t render the objections under section 3 (d) of the Patents Act in the first examination report and as well as in the hearing notice. The objection was notified for the first time during the date of hearing only.
Box 2: The section 3d points that “the mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or apparatus unless such known process results in a new product or employs at least one new reactant”.
The IPAB released an order dated 31st October 2014 to reconsider entire matter afresh by some other Assistant Controller, Patents and Designs other than the Assistant Controller who passed the orders impugned As per IPAB directions, this case was taken up once again by another Assistant Controller, Patents and Designs, Bharat N.S.
In the hearing, Pfizer stated that WO0142246 is not a prior art for the current invention since the prior art was published after priority date of the present application. Therefore the present application cannot be rejected based on the section 3d of THE PATENTS ACT, 1970.
However It was stated by the assistant controller that that WO0142246 can be considered for anticipation by previous publication and by prior claim based on the section 13(1)(b).
Box 3: The section 13(1)(b) states that “the invention so far as is claimed in any claim of any other complete specification published on or after the date of filing of the applicant’s complete specification, being a specification filed in pursuance of an application for a patent made in India and dated before or claiming the priority date earlier than that date”.
Thus, the application was rejected stating that ”an application for Patent filed at the Indian Patent Office before the date of filing of complete specification of a later filed application but published after the same is considered for the purposes of prior claiming”.
The inventive compound in the rejected application is the enantiomer of the prior art compound. Due to lack of proof for improved therapeutic efficacy over the compound claimed in the prior art, Pfizer’s patent application refused by controller for the second time on September 03, 2015.
Pfizer is reviewing options for further action. IP world is watching the further progress in this case, which is similar to Novartis case on Gleevec which was rejected by Supreme Court of India on April 01, 2013 under section 3d & 3b of THE INDIAN PATENTS ACT, 1970. In both the cases, IPO rejected the grant of patent stating that mere discovery / improvement of known compound cannot be patented unless it has improved therapeutic efficacy.
However, Pfizer holds the exclusive right to market Tofacitinib since its base patent IN241773 is still in force in India. Further pointing to the unique standards of India, Pfizer’s patent application claiming the new chemical formulation has been granted in countries like Czech Republic, Croatia, New Zealand and Philippines.
India’s high standards to grant patents has for sure sent across the message to pharma companies especially, that proof for improved therapeutic efficacy is a must for any new form of known compound before filing a patent in India.
These judgements would boost pharma companies to give extra care on therapeutic efficacy while finding new form of known compound, which in turn, will be a boon for people who are suffering from debilitating disorders.
This blog is contributed by Sujatha and Dr. Lalitha, patent scientists at DexPatent. Published on 13th October, 2015.
For any comments/ feedbacks please write to info@dexpatent.com or PatentScientist@dexpatent.com









0 Comments